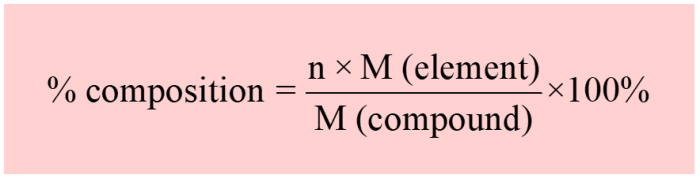Electron energy and light pogil answers, a topic that intertwines the fundamental principles of physics and chemistry, unveils a fascinating world where light and matter engage in a dynamic interplay. This guide delves into the intricacies of electron energy levels, the quantization of light, and the applications that harness these concepts.
Delving deeper into the topic, we explore the energy level structure of atoms, the mechanisms of electron transitions, and the significance of electron energy in chemical reactions and spectroscopy. Furthermore, we uncover the processes of light emission and absorption, shedding light on the principles behind lasers, LEDs, photovoltaics, and photodetectors.
Energy of Light
Light is a form of electromagnetic radiation. Electromagnetic radiation is made up of photons, which are particles of light. The energy of a photon is proportional to its frequency. The higher the frequency of a photon, the higher its energy.
The relationship between the energy of a photon and its wavelength is given by the equation E = hc/λ, where E is the energy of the photon, h is Planck’s constant, c is the speed of light, and λ is the wavelength of the photon.
The energy of light is quantized, which means that it can only exist in discrete packets. The size of these packets is determined by the frequency of the light. The higher the frequency of the light, the smaller the size of the packets.
The energy of light is used in a variety of practical applications. For example, light is used to generate electricity in solar cells, to create images in cameras, and to transmit information in fiber optic cables.
Electron Energy Levels
Atoms are made up of a nucleus and electrons. The nucleus is located at the center of the atom and contains protons and neutrons. The electrons are located in shells around the nucleus. Each shell has a specific energy level.
The energy level of a shell increases as the distance from the nucleus increases.
Electrons can transition between energy levels by absorbing or emitting photons. When an electron absorbs a photon, it moves to a higher energy level. When an electron emits a photon, it moves to a lower energy level.
The energy of the photon that is absorbed or emitted is equal to the difference in energy between the two energy levels.
Electron energy levels are involved in a variety of chemical reactions and spectroscopy. For example, the energy of the electrons in a molecule determines the molecule’s chemical reactivity. The energy of the electrons in an atom can be used to identify the atom.
Electron Energy and Light Emission, Electron energy and light pogil answers
When an electron is excited to a higher energy level, it can emit a photon of light as it returns to a lower energy level. The wavelength of the emitted light is determined by the energy difference between the two energy levels.
The process of light emission by excited atoms is used in a variety of applications, such as lasers and LEDs. Lasers are used to produce a concentrated beam of light. LEDs are used to produce light in electronic devices.
Electron Energy and Light Absorption
When an atom absorbs a photon of light, the energy of the photon is used to excite an electron to a higher energy level. The wavelength of the absorbed light is determined by the energy difference between the two energy levels.
The process of light absorption by atoms is used in a variety of applications, such as photovoltaics and photodetectors. Photovoltaics are used to convert light into electricity. Photodetectors are used to detect light.
Helpful Answers: Electron Energy And Light Pogil Answers
What is the relationship between electron energy and light?
Electrons can absorb or emit photons, resulting in transitions between energy levels. The energy of the photon corresponds to the energy difference between the electron’s initial and final energy levels.
How does light emission occur?
When an excited atom returns to a lower energy level, it releases a photon with energy equal to the difference between the two energy levels.
What are the applications of electron energy and light absorption?
Electron energy and light absorption find applications in photovoltaics, photodetectors, and spectroscopy, among others.