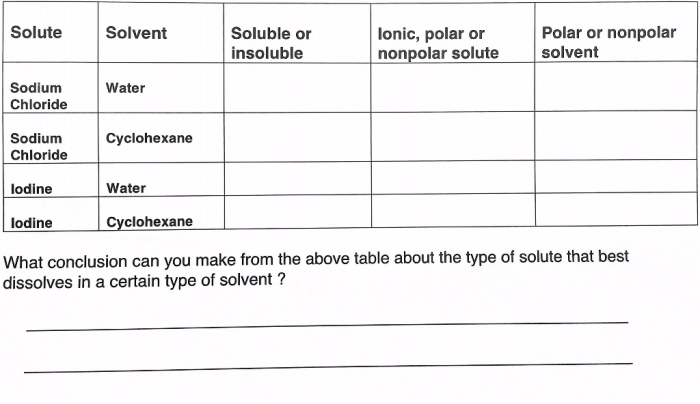
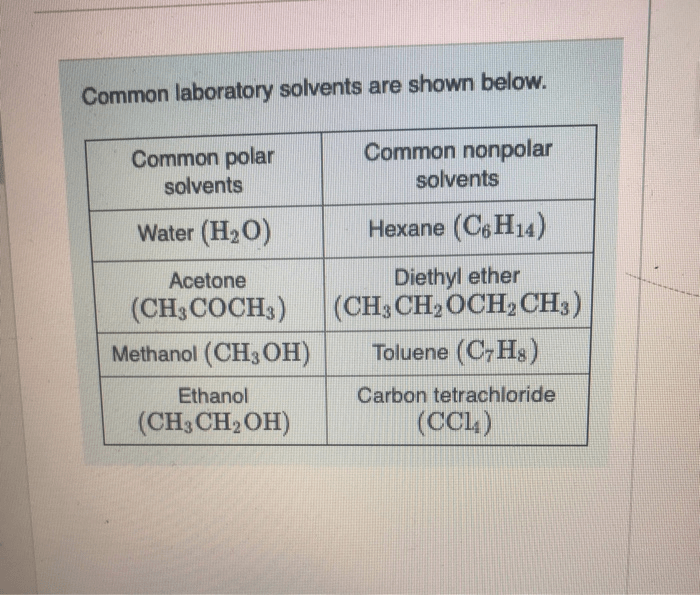
The topic of ions in polar solvents quick check has garnered significant attention in recent times, attracting the interest of researchers and practitioners alike. This comprehensive guide delves into the intricacies of ion solvation, exploring the fundamental concepts, key interactions, and practical applications associated with this phenomenon.
Within the realm of chemistry, ions in polar solvents play a pivotal role, influencing a wide range of chemical processes and reactions. Understanding the behavior of ions in these solvents is crucial for advancing our knowledge in various scientific disciplines.
General Inquiries: Ions In Polar Solvents Quick Check
What is ion solvation?
Ion solvation refers to the process by which ions interact with and become surrounded by solvent molecules in a polar solvent.
How does solvent polarity affect ion solvation?
The polarity of a solvent plays a crucial role in ion solvation. Polar solvents, which have a separation of charge, can effectively solvate ions by aligning their dipoles to minimize electrostatic interactions.
What are the different types of ion-solvent interactions?
Ion-solvent interactions can be classified into various types, including electrostatic interactions, hydrogen bonding, and van der Waals forces. These interactions influence the solvation energy and behavior of ions in solution.