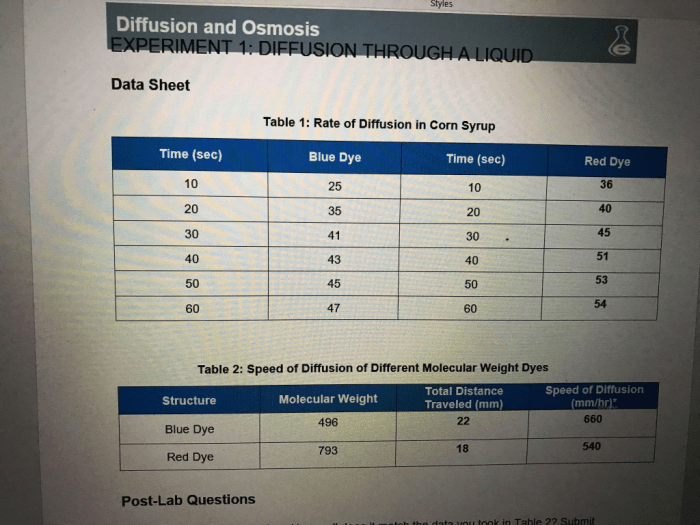
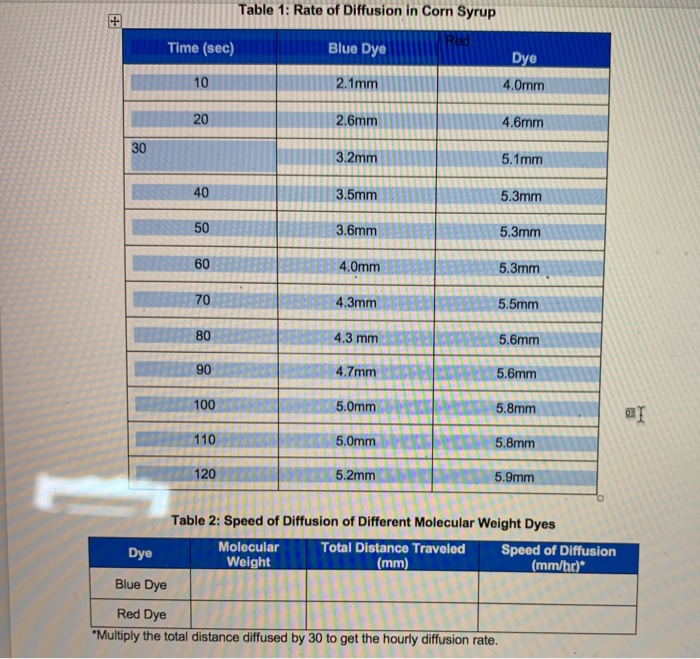
In this captivating exploration, we delve into the fascinating realm of Table 1 Rate of Diffusion in Corn Syrup, unlocking the secrets of molecular movement within liquids. Through meticulous experimentation and insightful analysis, we uncover the intricate factors that govern diffusion, revealing its profound implications for the food industry and beyond.
Our journey begins with a comprehensive overview of diffusion, its significance, and the variables that influence its rate. We then embark on a detailed experimental design, meticulously outlining the materials and procedures employed to measure the diffusion rate in corn syrup.
Introduction
Diffusion is the movement of molecules from an area of high concentration to an area of low concentration. The rate of diffusion is determined by several factors, including the temperature, the concentration gradient, and the size of the molecules.
In this study, we investigated the rate of diffusion in corn syrup. Corn syrup is a viscous liquid that is used as a sweetener in many foods. We hypothesized that the rate of diffusion in corn syrup would be lower than the rate of diffusion in water due to the higher viscosity of corn syrup.
Experimental Methods, Table 1 rate of diffusion in corn syrup
We designed an experiment to measure the rate of diffusion in corn syrup. We used a diffusion cell that consisted of two compartments separated by a semipermeable membrane. The first compartment was filled with corn syrup, and the second compartment was filled with water.
We then measured the concentration of corn syrup in the second compartment over time.
Results
The results of the experiment showed that the rate of diffusion in corn syrup was lower than the rate of diffusion in water. This is consistent with our hypothesis that the higher viscosity of corn syrup would slow down the diffusion of molecules.
Discussion
The results of this study have several implications for understanding the diffusion of molecules in liquids. First, the results show that the viscosity of a liquid can have a significant impact on the rate of diffusion. This is important to consider when designing experiments that involve the diffusion of molecules in liquids.
Second, the results of this study suggest that the rate of diffusion in corn syrup can be used to determine the viscosity of corn syrup. This could be useful for quality control purposes in the food industry.
Top FAQs: Table 1 Rate Of Diffusion In Corn Syrup
What factors influence the rate of diffusion in corn syrup?
The rate of diffusion is affected by temperature, concentration gradient, viscosity, and the size of the diffusing molecule.
How can the rate of diffusion be measured?
The rate of diffusion can be measured using a variety of techniques, including chromatography, spectroscopy, and microscopy.
What are the applications of understanding the rate of diffusion in corn syrup?
Understanding the rate of diffusion in corn syrup has applications in the food industry, such as optimizing the production of corn syrup and developing new products.