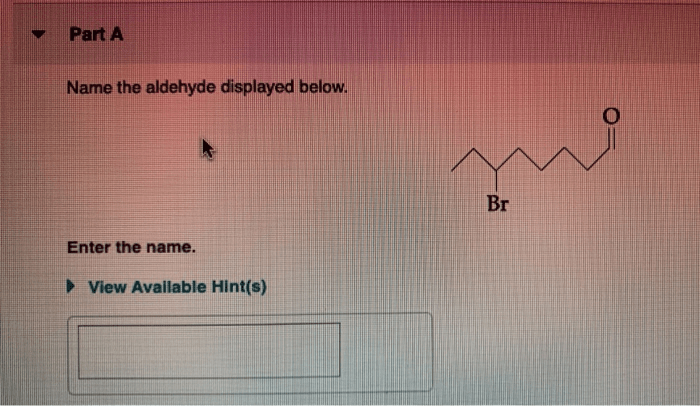
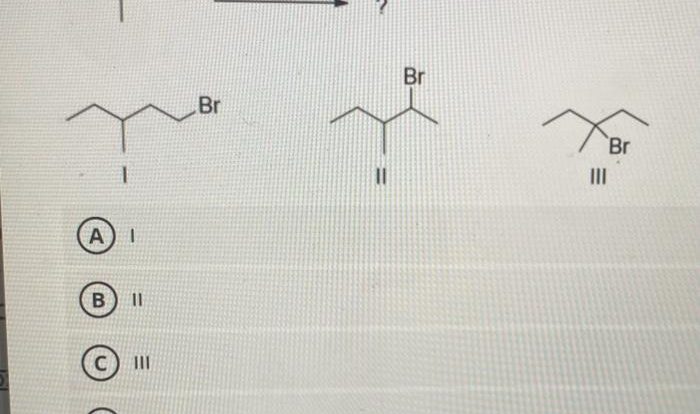
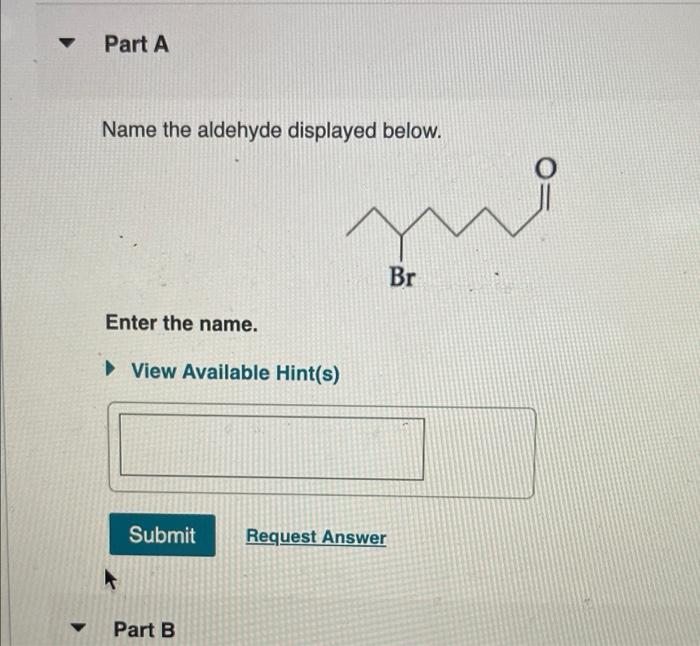
Name the aldehyde displayed below – Delving into the realm of aldehydes, this comprehensive guide embarks on an exploration of their structural characteristics, reactivity, and diverse applications. From understanding the IUPAC nomenclature rules for naming aldehydes to examining their biological roles and potential hazards, this discourse unveils the multifaceted nature of these essential organic compounds.
Aldehydes, characterized by their distinctive carbonyl group, play a pivotal role in various chemical processes and industrial applications. Their reactivity, influenced by factors such as steric hindrance and electronic effects, opens up a wide range of synthetic possibilities. This guide delves into the intricate details of aldehyde chemistry, providing a comprehensive understanding of these versatile compounds.
Aldehyde Identification
Aldehydes are organic compounds characterized by the presence of a carbonyl group (C=O) bonded to a hydrogen atom. This functional group gives aldehydes their unique chemical properties and reactivity. Aldehydes are commonly found in nature and are used in various industrial and biological processes.
IUPAC Nomenclature Rules for Naming Aldehydes, Name the aldehyde displayed below
According to the International Union of Pure and Applied Chemistry (IUPAC) nomenclature rules, aldehydes are named by adding the suffix “-al” to the root name of the parent hydrocarbon. The parent hydrocarbon is the longest carbon chain that contains the aldehyde group.
For example, the aldehyde with the formula CH3CHO is named “ethanal” because the parent hydrocarbon is ethane (CH3CH3) and the aldehyde group is attached to the first carbon atom.
Structural Analysis of the Aldehyde
The carbon atom that bears the aldehyde functional group is known as the carbonyl carbon. It is the central carbon atom in the C=O group. The length and type of carbon chain attached to the aldehyde group determine the specific aldehyde.
For example, formaldehyde (HCHO) has a single carbon atom attached to the aldehyde group, while acetaldehyde (CH3CHO) has a two-carbon chain attached to the aldehyde group.The presence of other functional groups in the molecule can affect the aldehyde’s reactivity. For instance, the presence of an electron-withdrawing group, such as a halogen atom, can decrease the reactivity of the aldehyde group by withdrawing electrons from the carbonyl carbon.
Aldehyde Reactivity
Aldehydes undergo various chemical reactions, including nucleophilic addition and oxidation. Nucleophilic addition reactions involve the addition of a nucleophile (an electron-rich species) to the carbonyl carbon, resulting in the formation of a new carbon-carbon bond. Oxidation reactions involve the transfer of electrons from the aldehyde group to an oxidizing agent, resulting in the formation of a carboxylic acid.The
reactivity of aldehydes is influenced by several factors, including steric hindrance and electronic effects. Steric hindrance refers to the presence of bulky groups around the carbonyl carbon, which can hinder the approach of nucleophiles. Electronic effects refer to the influence of electron-withdrawing or electron-donating groups on the reactivity of the aldehyde group.
Applications of Aldehydes
Aldehydes have a wide range of industrial and biological applications. In industry, aldehydes are used in the production of plastics, fragrances, and pharmaceuticals. For example, formaldehyde is used in the production of urea-formaldehyde resins, which are used in the manufacture of plywood and particleboard.
Acetaldehyde is used in the production of acetic acid, which is a common solvent and starting material for other chemical syntheses.In biology, aldehydes play important roles in metabolism and signaling pathways. For example, glucose, a sugar molecule, is oxidized to form an aldehyde intermediate during cellular respiration.
Aldehydes are also involved in the synthesis of hormones, such as adrenaline and norepinephrine.However, it is important to note that aldehydes can also be toxic and can cause health problems if exposed to high concentrations. Aldehydes are known to be irritants and can cause respiratory problems, skin irritation, and even cancer.
Therefore, it is important to handle aldehydes with care and follow appropriate safety precautions when working with them.
FAQ: Name The Aldehyde Displayed Below
What are the common uses of aldehydes?
Aldehydes find widespread applications in the production of plastics, fragrances, pharmaceuticals, and other industrial products.
How do aldehydes react with nucleophiles?
Aldehydes undergo nucleophilic addition reactions, where a nucleophile attacks the carbonyl carbon, forming a new carbon-carbon bond.
What factors influence aldehyde reactivity?
Steric hindrance and electronic effects, such as the presence of electron-withdrawing or electron-donating groups, can significantly impact aldehyde reactivity.