Acetic acid and 3 methyl 1 butanol – Acetic acid and 3-methyl-1-butanol are versatile organic compounds with diverse industrial and laboratory applications. This overview explores their chemical structures, physical and chemical properties, production methods, reactivity, and practical uses, providing a comprehensive understanding of these essential substances.
Introduction
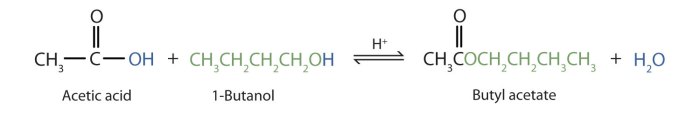
Acetic acid and 3-methyl-1-butanol are two important organic compounds with a wide range of industrial and laboratory applications. Acetic acid, also known as ethanoic acid, is a colorless liquid with a pungent odor. It is a weak acid that is miscible with water and many organic solvents.
3-Methyl-1-butanol, also known as isoamyl alcohol, is a colorless liquid with a banana-like odor. It is a primary alcohol that is insoluble in water but miscible with most organic solvents.
Physical and Chemical Properties
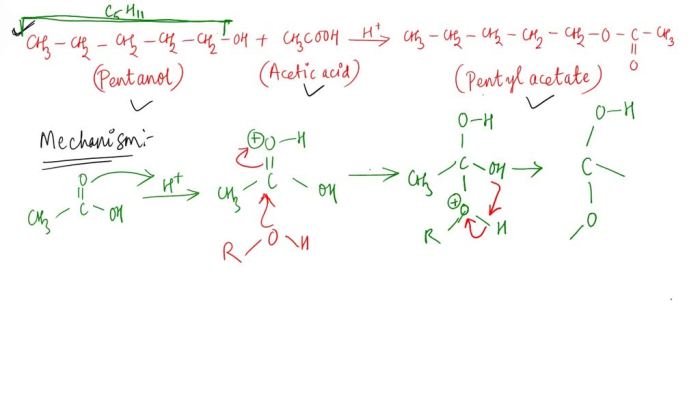
The physical and chemical properties of acetic acid and 3-methyl-1-butanol are compared in the following table:
| Property | Acetic acid | 3-Methyl-1-butanol |
|---|---|---|
| Molecular weight | 60.05 g/mol | 88.15 g/mol |
| Boiling point | 118.1 °C | 131.7 °C |
| Melting point | 16.6 °C | -117 °C |
| Density | 1.049 g/mL | 0.802 g/mL |
| Solubility in water | Miscible | Insoluble |
| Acidity (pKa) | 4.76 | 15.6 |
Production and Synthesis
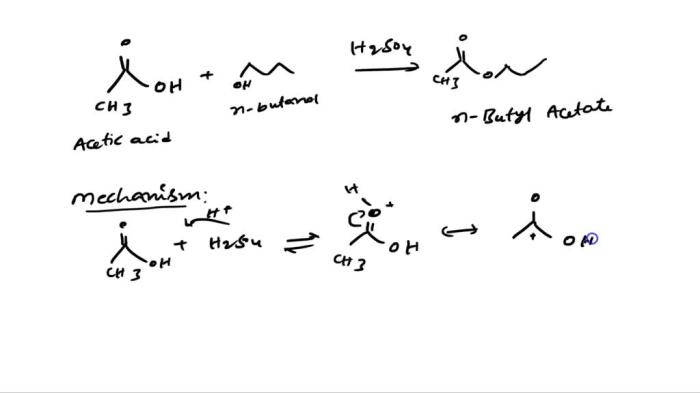
Acetic acid is produced industrially by the fermentation of ethanol. It can also be synthesized from methanol and carbon monoxide in a process known as the Monsanto process.
3-Methyl-1-butanol is produced industrially by the hydration of isobutylene. It can also be synthesized from acetone and hydrogen in a process known as the Guerbet reaction.
Query Resolution: Acetic Acid And 3 Methyl 1 Butanol
What is the difference between acetic acid and 3-methyl-1-butanol?
Acetic acid is a carboxylic acid, while 3-methyl-1-butanol is an alcohol. Acetic acid has a pungent odor and is corrosive, while 3-methyl-1-butanol has a sweet odor and is less corrosive.
How are acetic acid and 3-methyl-1-butanol produced?
Acetic acid is primarily produced by the fermentation of ethanol, while 3-methyl-1-butanol is produced by the hydration of isobutylene.
What are the applications of acetic acid and 3-methyl-1-butanol?
Acetic acid is used in the production of vinegar, plastics, and pharmaceuticals, while 3-methyl-1-butanol is used as a solvent, in the production of fragrances, and as a fuel additive.